Clinical Translation and Applications of Cancer Nanomedicine: A Systematic Review
Keywords:
- Cancer nanomedicine, nanoparticles, clinical trials, drug delivery, liposomes, biomimetic carriers, regulatory approvals, personalized oncology
Abstract
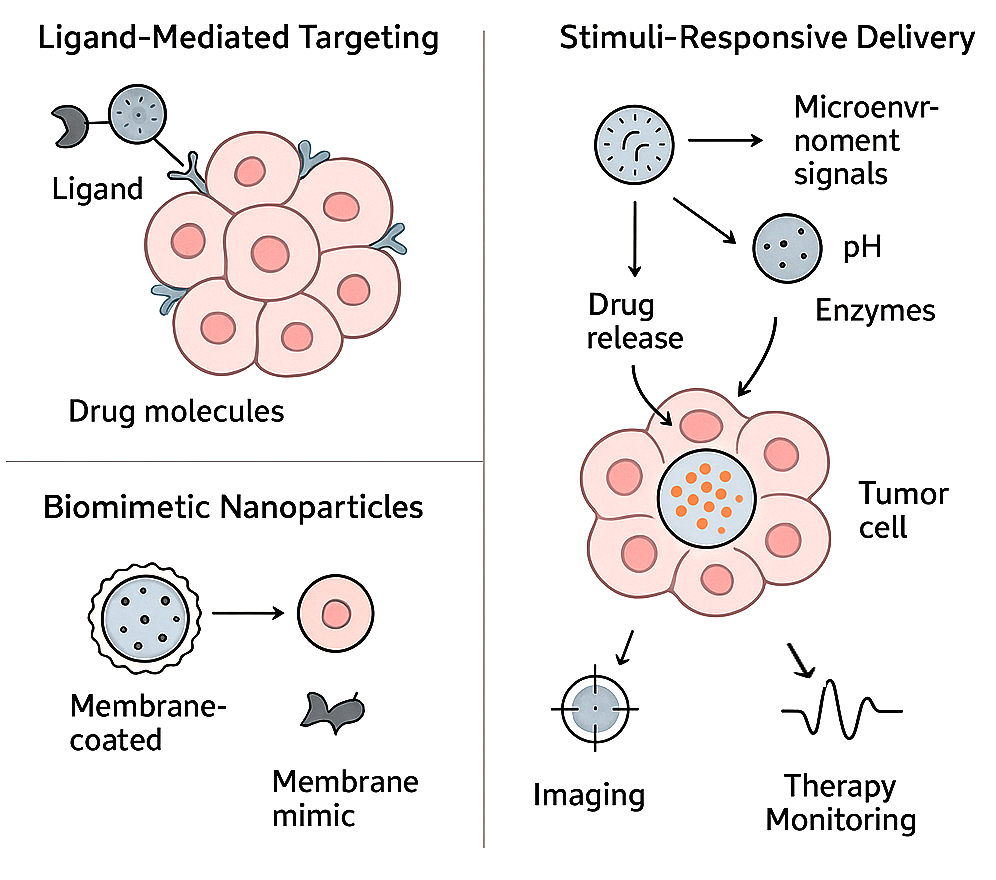
Background: Cancer nanomedicine has emerged as a transformative paradigm that harnesses nanoscale platforms to enhance drug delivery, imaging, and patient outcomes. Between 2019 and 2024, remarkable advances have accelerated the clinical translation of nanomedicine, bridging experimental innovations with bedside applications. Objective: This systematic review evaluates the clinical applications of cancer nanomedicine reported from 2019 to 2024, with emphasis on technological innovations, translational challenges, regulatory approvals, real-world outcomes, and future prospects. Methods: A comprehensive literature search was conducted across PubMed, Scopus, Web of Science, and ClinicalTrials.gov, supplemented by regulatory databases. Inclusion criteria focused on clinical trials, preclinical studies with translational impact, regulatory approvals, and real-world data involving cancer nanomedicine. The PRISMA 2020 framework guided study selection, and methodological quality was assessed using validated tools. Results: A were included. Liposomes, dendrimers, polymeric nanoparticles, albumin-bound formulations, and biomimetic nanocarriers dominated the landscape, offering improved pharmacokinetics, targeted delivery, and reduced systemic toxicity. Clinical trials demonstrated efficacy across multiple malignancies, including breast, ovarian, lung, pancreatic, and hematological cancers. Regulatory approvals for agents such as nab-paclitaxel, liposomal irinotecan, and Vyxeos reinforced the clinical relevance of nanomedicine. Real-world data confirmed superior safety, reduced cardiotoxicity and neuropathy, and improved quality of life compared to conventional therapies. However, translational challenges nanotoxicity, scalability, cost, and regulatory complexityremain significant barriers. Conclusion: Cancer nanomedicine has progressed from conceptual innovation to a clinically validated reality, reshaping therapeutic strategies across oncology. Integration with immunotherapy, gene therapy, and AI-driven design promises to overcome current limitations, paving the way for truly personalized and precision-based cancer care.