Translational Nanomedicine in Oncology: AI-Driven Multistage Targeting Strategies for Liver, Breast, Kidney, and Brain Cancers
Keywords:
- Nanomedicine, Cancer therapy, Liver cancer, Breast cancer, Kidney cancer, Brain cancer, Multistage targeting, Biomimetic nanoparticles, Stimuli-responsive drug delivery, Theranostics, Artificial intelligence, Microfluidics, Patient-specific oncology, Translational nanomedicine, Precision oncology
Abstract
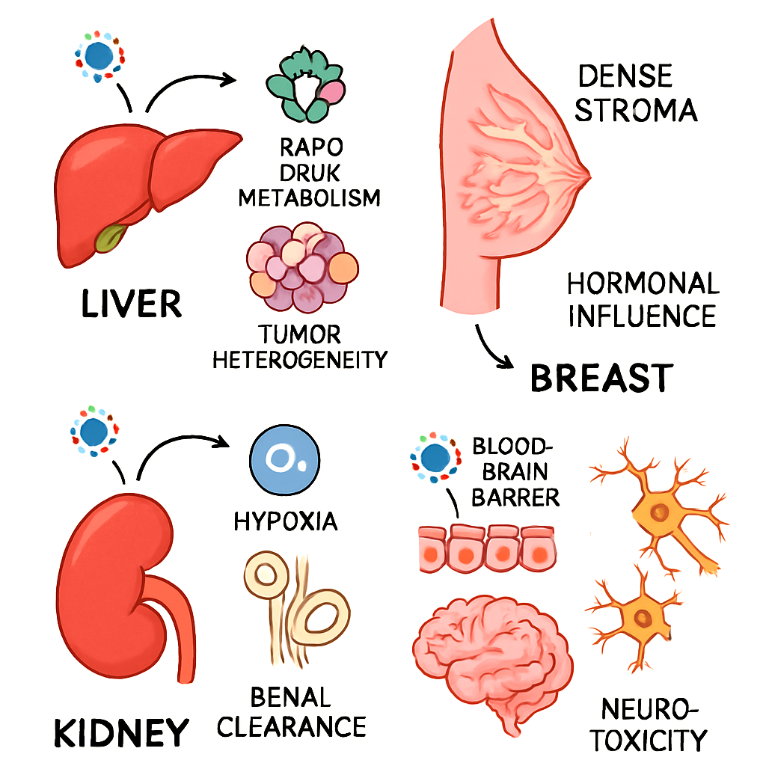
Cancer continues to rank among the leading causes of global mortality, with liver, breast, kidney, and brain malignancies presenting some of the most complex therapeutic challenges. Conventional treatments such as chemotherapy, radiotherapy, and targeted agents are constrained by tumor heterogeneity, systemic toxicity, and the emergence of drug resistance.Aim:
This systematic review evaluates advancements in nanomedicine between 2019 and 2024, focusing on translational innovations for liver, breast, kidney, and brain cancers. Special emphasis is placed on preclinical breakthroughs, clinical trial outcomes, and the integration of artificial intelligence (AI) and microfluidics in developing patient-specific therapeutic platforms. Methods:
Following PRISMA guidelines, an extensive literature search was conducted across PubMed, Scopus, Web of Science, and ClinicalTrials.gov. Eligible studies included preclinical models, phase I–III clinical trials, and translational research on nanomedicine-based interventions for the selected cancers. Data were extracted on nanocarrier type, targeting strategy, and mechanism of action, efficacy, safety, and translational readiness. AI-driven design approaches and microfluidics-enabled synthesis platforms were also analyzed for their role in accelerating optimization and personalization. Result:Preclinical studies demonstrated the efficacy of multistage targeting nanocarriers incorporating passive, active, and hierarchical mechanisms, as well as stimuli-responsive systems triggered by tumor microenvironmental cues (pH, enzymatic activity, and hypoxia). Biomimetic nanoparticles, including cell membrane-coated carriers, showed enhanced immune evasion and tumor homing. Clinically validated examples such as liposomal doxorubicin, nanoparticle albumin-bound paclitaxel, and receptor-targeted polymeric nanoparticles reported improved tumor response rates, reduced off-target toxicity, and favorable safety profiles. Theranostic platforms integrating imaging and therapy enabled real-time monitoring and adaptive treatment strategies. AI algorithms facilitated predictive modeling of nanoparticle–tumor interactions, optimization of ligand density, and payload release kinetics, while microfluidics ensured scalable, reproducible manufacturing and organ-on-chip-based preclinical validation.Conclusion:Nanomedicine has transitioned from experimental proof-of-concept to a viable clinical reality in oncology, offering unprecedented precision, adaptability, and multifunctionality. The integration of AI-driven design and microfluidic fabrication is accelerating the translation of nanocarriers into patient-specific therapies, with the potential to overcome long-standing barriers in drug delivery. These advances mark a paradigm shift toward data-driven, personalized nanomedicine, positioning it as a cornerstone of next-generation cancer care.